INDUSTRIAL TECHNOLOGY RESEARCH INSTITUTE
About us
ITRI ( Industrial Technology Research Institute )
ITRI is a not-for-profit R&D organization engaging in applied research and technical services. Founded in 1973, ITRI has played a vital role in transforming Taiwan's economy from a labor-intensive industry to a high-tech industry. They are also a world-leading applied technology research institute with more than 6,000 outstanding employees. Its mission is to drive industrial development, create economic value, and enhance social well-being through technology R&D.
ITRI is dedicated to creating new value and identifying emerging demands for partners while facing global challenges and opportunities, such as urbanization, aging societies, new production and consumption patterns, climate change caused by global warming, and a post-COVID-19 era.
PV Innovation Award
In order to encourage Taiwan photovoltaic industry to focus on innovation strengthen the integration capability of Green Energy system , and enhance international competitiveness. Since 2019, the MOEA commissioned the Industrial Technology Research Institute(ITRI) to hold the PV Innovation Award.
Through a fair and just review mechanism, the most representative Taiwanese Innovation Green Technology idea or product is selected.
ITRI ( Industrial Technology Research Institute )
ITRI is a not-for-profit R&D organization engaging in applied research and technical services. Founded in 1973, ITRI has played a vital role in transforming Taiwan's economy from a labor-intensive industry to a high-tech industry.e're also a world-leading applied technology research institute with more than 6,000 outstanding employees. Its mission is to drive industrial development, create economic value, and enhance social well-being through technology R&D.
ITRI is dedicated to creating new value and identifying emerging demands for partners while facing global challenges and opportunities, such as urbanization, aging societies, new production and consumption patterns, climate change caused by global warming, and a post-COVID-19 era.
PV Innovation Award
In order to encourage Taiwan photovoltaic industry to focus on innovation strengthen the integration capability of Green Energy system , and enhance international competitiveness. Since 2019, the MOEA commissioned the Industrial Technology Research Institute(ITRI) to hold the PV Innovation Award.
Through a fair and just review mechanism, the most representative Taiwanese Innovation Green Technology idea or product is selected.
Products
Technology Services
Joint Research
ITRI teams up with internationally renowned universities, R&D institutes or enterprises to conduct joint research to develop breakthrough foresight technologies.
Technology Transfer
ITRI introduces IPs that add value to ITRI’s existing technologies or IP portfolios to help upgrade Taiwan’s industries and strengthen their position in the market. ITRI transfers its technologies to domestic or international enterprises to meet their demand for internationalization.
Contract Service
ITRI undertakes strategic/industrial consulting services contracted by international enterprises. Projects as such encourage ITRI’s R&D units, domestic companies and industrial associations to stay current with global trends.
Testing and Verification
ITRI provides professional inspection, calibration, measurement, testing, and verification services. ITRI's testing laboratories meet international standards and inspection quality norms, and the test reports are considered highly trustworthy.
Pilot Production and Value-added Services
Prior to product commercialization, ITRI provides pilot production and value-added services, including manufacturing process verification, product development, and production of semi-finished products. Our partners can carry out pilot production on test production lines at ITRI, during which potential risks associated with mass production can be assessed in advanced.
Prototyping & Manufacturing
The objective of this service is to help business partners bridge the gap between R&D and creation of a final product. ITRI can provide services including prototyping and manufacturing, and encompass related issues such as reliability testing, integration of components, new product development, trial products, improving manufacturing processes, and work safety testing.
One-Stop Biomedical Services
ITRI is committed to driving the development of various industries with its outstanding technology and research abilities in hopes to create economic values and promote social well-being. In addition to playing a role of researcher and developer in the fields of medicine as well as medical devices, it has also established a complete structure of CRO (Contract Research Organizations) and CDMO (Contract Development & Manufacturing Organization) services. The one-stop biomedical services platform of ITRI provides assistance in a wide range of scopes, including research and development, model translation, GXP trial production, pre-clinical/clinical trial, post-marketing consultation, etc. This mechanism makes it possible for domestic manufacturers to lower their initial cost of investment, and accelerate their research and development.
- Listing Regulations Consulting: FDA/TFDA/CE application, medical device verification/export, medical device/medicine registration, IRB document organization, clinical trial application, and more.
- Technology Transfer: Bio-IT, diagnostics and precision medicine technology, regeneration medicine technology, natural medicine and healthcare technology, targeted drug and delivery technology.
- Model Translation: Domestic supply chain linkage, medical device design control, market analysis, strategy analysis of regulation in commercialization, and more.
- Preclinical / Clinical Trial: Functional safety assessments, clinical research organization linkage, clinical evaluation and analysis, clinical trial planning, clinical trial analysis and reports.
- Other Services: Quality system consulting, commercialization consulting, smart health solutions, software validation, medical device risk management, information security risk evaluation, technical documents preparation, business ecosystem linkage, and more
ITRI is committed to driving the development of various industries with its outstanding technology and research abilities in hopes to create economic values and promote social well-being. In addition to playing a role of researcher and developer in the fields of medicine as well as medical devices, it has also established a complete structure of CRO (Contract Research Organizations) and CDMO (Contract Development & Manufacturing Organization) services. The one-stop biomedical services platform of ITRI provides assistance in a wide range of scopes, including research and development, model translation, GXP trial production, pre-clinical/clinical trial, post-marketing consultation, etc. This mechanism makes it possible for domestic manufacturers to lower their initial cost of investment, and accelerate their research and development.
%20ITRI%20Web_One-Stop%20Services%20800x337.jpg)
- Listing Regulations Consulting: FDA/TFDA/CE application, medical device verification/export, medical device/medicine registration, IRB document organization, clinical trial application, and more.
- Technology Transfer: Bio-IT, diagnostics and precision medicine technology, regeneration medicine technology, natural medicine and healthcare technology, targeted drug and delivery technology.
- Model Translation: Domestic supply chain linkage, medical device design control, market analysis, strategy analysis of regulation in commercialization, and more.
- Preclinical / Clinical Trial: Functional safety assessments, clinical research organization linkage, clinical evaluation and analysis, clinical trial planning, clinical trial analysis and reports.
- Other Services: Quality system consulting, commercialization consulting, smart health solutions, software validation, medical device risk management, information security risk evaluation, technical documents preparation, business ecosystem linkage, and more.
Catalog
- Molecular Testing and Medical Device Testing Laboratory
- Medical Device GMP Manufacturer
- Quality Control Laboratory of Cell Manufacturing Facility
- Cell Medium Products GMP Plant
- Cell Manufacture Factory
- Biomaterial Product GMP Plant
- Animal Laboratory for Biomedical Research
- Laboratory of Cosmetics Microbial Testing and Functional Evaluation
- GMP Pilot Plant for Botanical Drug Products
- API PIC/S GMP Pilot Plant
- Aseptic Pharmaceutical Formulation Factory
- Regulatory Affairs Services
- Taiwan Integrated Biomedical Industrial Center (TIBIC)
Molecular Testing and Medical Device Testing Laboratory

Molecular Testing
Assist in upgrading laboratory results (RUO) to LDTs services, provide precision medical molecular testing LDTs service, and accelerate the commercialization process and benefits.
- ISO17025 certification and TFDA-LDTs list registration-two test items of mycoplasma and EGFR
- A core laboratory for molecular testing that meets the needs of international regulations
【Services】
- SARS-CoV2 molecular reagent product quality control test
- Counseling molecular laboratory construction planning and quality system construction
【Core Facility】
- Sample Pre-Processing System
Nucleic acid (DNA & RNA) extraction and purification of various clinical samples - Real-Time Nucleic Acid Amplification Detection System
Nucleic acid molecule amplification quantitative detection, mutation and gene expression analysis - Digital Nucleic Acid Amplification and Detection System
High-sensitivity digital nucleic acid molecule amplification and quantitative detection - Micro Nanoparticle Analysis System
Cell Exosomes Analysis - Gene Sequencing Analysis System
Gene sequencing, mutation and gene expression analysis
【Contact】
- Mr.Liu: +886-3-591-9139|E-mail: williamliu@itri.org.tw

Medical Device Testing
Assist the industry in material evaluation, provide third-party verification reports from domestic blood glucose meter manufacturers, accelerate product development progress, and provide credible verification reports from foreign buyers.
- Obtained TAF ISO17025 initial certification in April 2018
Certification items: ISO15197 6.2.3 repeatability test and ISO15197 6.2.3 intermediate precision test
【Services】
- Blood glucose test strip material detection or related electrochemical detection technology assistance
- Counseling laboratory construction planning and TAF ISO17025 quality system construction
【Core Facility】
- YSI 2300 Brix Tester, Refrigerated high-speed centrifuge, Capillary centrifuge, Roche Biochemical Analyzer, Constant temperature and humidity meter, Laser engraver, Electrochemical Workstation, Microdispensor
【Contact】
Miss Chen +886-3-591-9138|E-mail: EmilyChen@itri.org.tw
Medical Device GMP Manufacturer

The GMP process accumulates complete design and development documents and experience, and has high-quality medical device trial production and manufacturing technology to complete the GMP environment hardware implementation. Establish testing services and trial production lines to assist in the verification of new technologies and cross the last mile. Promote small-scale trial production of innovative service products and assist in nurturing start-up companies.
- iKnobeads products obtained TFDA GMP registration (March, 2021)
- iPMx products (iPMx analyzer and covid-19 reagent kit) obtained TFDA project manufacturing approval (July, 2020)
- iPMx products (iPMx analyzer and covid-19 regaent kit) applied for Japanese inspection and registration (May, 2021)
- Obtained a medical device GMP factory manufacturer license (September 27, 2019)
- Obtained the GMP factory registration certificate (September 26, 2019)
- GMP factory environmental protection approval letter. (July 8, 2019)
- It has a quality system that has passed ISO13485. (April 2, 2019)
【Services】
- Counseling GMP quality system, (including design, manufacturing, packaging and shipping)
- Counseling plant design and planning
- Commissioned design and trial production
- Consignment manufacturing of medical electrical device
- Small batch mass production of IVD detection reagents or immunotherapy reagents and commissioned manufacturing
【Core Facility】
- It is mainly divided into medical electrical assembly plant, reagent sub-assembly plant, warehouse, and quality control laboratory.
Isolator, biosafety console, liquid chromatography analyzer, flow cytometer, particle counting and particle size analyzer, spectrophotometer, automatic cell counter, precision balance, refrigeration equipment, oscilloscope, power supply, etc.
【Contact】
Mr.Chang +886-3-591-3730|E-mail: Caleb.chang@itri.org.tw
Quality Control Laboratory of Cell Manufacturing Facility
We are the professional laboratory in Taiwan that provides quality control services of cell product manufacturing, including raw materials, intermediates and final products. We also provide contract research services for cell product inspection.
- The lab is certified on ISO/IEC 17025 and has eight test abilities.
【Services】
Quality Control Inspection Services
- ISO/IEC 17025 and TAF certification
-Sterility and Bacteriostasis/Fungistasis Tests by Direct or Membrane Filtration Method
-Endotoxin Test and Inhibition/Enhancement Test for Endotoxin by Gel Clotting Method
-Mycoplasma test by PCR Method
- Cell Surface Antigen Analysis
- Automated Sterility Test (Automated Blood Culture System) (Compliance with Pharmacopoeia 7021)
- Consultation on Related Testing Items
【Core Facility】
- Automated sterility testing , ABI Veriti PCR machine, Three Low Temperature Incubator, Constant Temperature Water Bath, The Pyros Kinetix® Flex
【Contact】
Mr. Huang +886-3-591-7898|E-mail: Sigher@itri.org.tw
Cell Medium Products GMP Plant

Adhering to the spirit of continuous innovation of the Industrial Technology Research Institute, and accumulating the long-term culture medium research and development experience and production of the R&D team, the ITRI culture medium product GMP plant is the first medium product plant to pass Taiwan's second-class medical material GMP certification. Our serum-free medium can be used to produce clinical grade cells for use in human cell therapy. Provide important key raw materials for Taiwan's biotechnology industry, breaking through the dilemma of long-term dependence on foreign imported culture media.
- Industrial Technology Research Institude Cell Medium Products GMP Plant is the only is the only serum-free culture media factory with a second-class medical material approval registration in Taiwan.
- The culture medium manufactured by Industrial Technology Research Institude Cell Medium Products GMP Plant is the only medium can produce clinical-grade cells in Taiwan (including the medium that are imported from abroad).
【Services】
- Customized commissioned serum-free medium research and development and mass production process development.
- Customized commissioned serum-free medium production.
- Purification of serum-free mesenchymal stem cells and transfer of high-efficiency in vitro proliferation technology.
【Core Facility】
- Aseptic semi-automatic filling machine with Class 100
【Contact】
Mr.Wang +886-3-591-2815|E-mail: ikwang@itri.org.tw
Cell Manufacture Factory
We are the first GTP facility in Taiwan offering cell production services. We have successfully assisted many customers establishing their GTP facilities and quality management systems. We provide contract service of culture room rental, product manufacturing, and process development.
- TFDA announced the Good Tissue Practice (GTP) standard operation (December 13, 2002)
- Factory registration certificate (November 8, 2007)
- Three QC Inspection tests have also passed TAF ISO/IEC 17025 certification (October 2, 2015)
【Services】
- GTP Service
We consultate the manufacturing to optimize the cell therapy products for clinical trials. We also provide GTP training courses, and we also provide the rental service of dedicated culture room. - QC Testing Service
ISO/IEC 17025, TAF-certified sterility testing, endotoxin testing and mycoplasma testing - Cell Storage in Liquid Nitrogen
Provide temporary storage of cell products produced in the ITRI CMF - Customized Service
Regulatory consultation, plant design consultation, quality control validation services, assistance in pilot testing, method evaluation and validation, and product manufacturing
【Core Facility】
- Self-balancing program incubator, automatic control of liquid nitrogen storage, program cooling instrument, automatic cell counting instrument with data preservation function, ultra-low temperature refrigerator, clean room air conditioning system linked with monitoring system, emergency generator, air-cooled ice water machine
【Contact】
Mr. Huang +886-3-591-7898|E-mail: Sigher@itri.org.tw
Biomaterial Product GMP Plant
Technical added-value: Our plant has met TFDA GMP quality system requirement. We are able to assist our clients in pre-clinical trial design and experiment to speed up product marketing processing.
Industry support: We provide assistance for domestic manufacturers on pilot trial medical production design and development, help to reduce on cost and risk management.
Talent training: Training of the implant medical device skillset including product development and manufacturing to increase the added value on the developmnt of medical device industry.
- Establish ASTMF2255、F2256、F2258 Validation processes of tissue sealing manufacturing and evaluation
- Establish ISO13485:2016、TFDA GMP、EU CE93/42/EEC、US FDA QMS quality system
- Four manufacutring facilities and two material preparation rooms for proudct pilot production manufacturing and evalution
【Services】
- GMP service
- Provide pilot trial production on type III implant medical devices
- Provide assistance on pre-clinical trial experiments for industry and medical institutes
- Provide assistance on product development of implantable medical device in compliance with ISO regulations to reduce cost and initial investment
- Focus on satisfying unmed medical needs and research
- Customized service
- Regulation consultation, Factory plant design consultation, Quality validation service, QC evalution and validation, OEM
【Core Facility】
- Class 10,000 clean room, RABs system, Static Hydraulic Industrial Series (Instron)
【Contact】
David Hung +886-3-591-8341|E-mail: itriA60244@itri.org.tw
Animal Laboratory for Biomedical Research

The Animal Laboratory for Biomedical Research (ALBR) was established to support the development of the biotechnology industry in accordance with national policies and invest in research and development in the fields of biotechnology, pharmaceutical industry, and medical device for promoting the research and development level of domestic academia and related industries. It mainly support the needs of the research for biomedicine and medical materials related programs within the Industrial Technology Research Institute (ITRI), and provides high-quality breeding environments and related technologies for laboratory animals.
- AAALAC certification (Since 2011)
- ISO/IEC 17025 accredited testing laboratory (Since 2010)
【Services】
- In addition to providing animal breeding services, it also provides preclinical trials and technical platforms for drug development, such as efficacy evaluation of various disease animal models, safety testing, pathological interpretation, blood and serum biochemical testing, etc.
【Core Facility】
- ALBR is divided into public areas, pathological and biochemical analysis laboratories and three animal experiment areas according to functions, including general animal experiment area, clean barrier area and ABSL-2 animal experiment area. The districts maintain environmental isolation from each other, and the access control is independent. It is also equipped with a non-invasive ln Vivo Imaging System ( IVIS®) for experimental needs.
【Contact】
Miss Liang +886-3-574-3947|E-mail: CYLiang@itri.org.tw
Laboratory of Cosmetics Microbial Testing and Functional Evaluation
A TAF accredited laboratory (Since 2007) provides the testing services including assessment for skin melanin, viscoelasticity, roughness, transepidermal water loss, moisture content of the skin surface. It provides professional services for cosmetics companies to evaluate cosmetic functions and develop functional skincare products.
【Services】
- TAF Accredited Items: Melanin content, Viscoelasticity, Transepidermal water loss, Moisture content of skin surface
- Efficacy evaluation of cosmetic products on skin melanin, skin elasticity and moisturizing effect.
【Core Facility】
- Courage+Khazaka electronic Cutometer MPA580
【Contact】
Mr. Gau +886-6-693-9089|E-mail: rjgau@itri.org.tw
GMP Pilot Plant for Botanical Drug Products

The first one pilot plant for botanical drugs with international ICH standards and official GMP certification among all of foundations in Taiwan. The rapid and systematic service platform for pilot run and quality control. It can be used for the development and application of botanical drugs and health products, and establish a scientific and systematic technology platform, which can accelerate the process of clinical translation and commercialization.
【Services】
- Optimization, scale-up, and standardization of process
- Test article preparation for non-clinical and early phase clinical trial
- Identification for botanical resource ingredients and Production of standards
- Analytical method development and validation
- Stability study of the production
- CMC documents construction
- Coaching for health food certification
- Coaching for GMP
【Contact】
Mr. Hsu +886-3-573-2606|E-mail: aspirin@itri.org.tw
API PIC/S GMP Pilot Plant

The pilot plant is funded by MOEA and ITRI, which becomes a PIC/S GMP inspected and qualified by TFDA. Through ITRI’s expertise team, we can offer customized products for preclinical and clinical use, this can shorten the development schedule in chemical synthesis and pharmaceutical mass production as well as the development of pharmaceutical analytics. In addition, the package services related with regulation science and PIC/S GMP consultation are provided.
- 1997, API cGMP pilot plant was build and manufactur for API
- 2001, equipments were validated and run for manufacuring
- 2020, PIC/S GMP from TFDA approval
【Services】
- GXP One-Stop Service platform: drug synthesis development and pilot manufacturing, analytical method development service platform, and GXP consulting service
【Core Facility】
- 10-500 L Reactor, Class B and C cleaning room, sterile manufacturing room, quality control lab and P2 lab
【Contact】
Dr. Cheng +886-3-573-2608|E-mail: sallyroseling@itri.org.tw
Dr. Lin +886-3-574-3987|E-mail: FoxLin@itri.org.tw
Aseptic Pharmaceutical Formulation Factory

Complex drug product attracts more attention in pharmaceutical industry ascribing the high entry barriers having few players. ITRI’s aseptic pharmaceutical formulation team has profound experiences in formulation design, analytic methods establishment, scale-up manufacturing process development and customized production line design for complex drug products. Our services aim to speed up product development and commercialization of complex formulation drug and bring benefits to the pharmaceutical industry.
【Services】
- Formulation development:
- To assist manufacturers to develop the best formula through the Design of Experiments (DOE)
- Amplified Manufacturing and Analytical Method development:
- Microspheres manufacturing
- Liposomes manufacturing
- Sterile liquid manufacturing
- Optimized Lyophilization process development
【Core Facility】
- Manufacturing instrument:
- Microspheres manufacturing
- High pressure homogenizer
- In-line homogenizer
- Spray dryer
- Isolator
- Automatic filling machine
- Tabletop filling machine
- Freeze dryer
- Analytical instrument:
- Chromatography (LC-UV、VIS、ELSD、GC)
- Particle size analyzer(nano grade、micro grade)
- Spray particles analyzer
- Differential scanning calorimeter
- Viscometer
- Osmometers
- Dissolution machine(USP dissolution Apparatus 1、2、4)
【Contact】
Dr. Felice Cheng +886-3-574-3973|E-mail: itriA00563@itri.org.tw
Dr. Yu-Wen Lo +886-3-574-3976|E-mail: itri950682@itri.org.tw
Regulatory Affairs Services
ITRI%20Web_Regulation%20800x533.jpg)
ITRI aims to assist the development of the medical device industry. Our experts are experienced in the medical device sector and specialized in regulatory strategies for companies to meet all the regulatory compliance requirements. We also offer consulting services with a holistic approach to lead your product go to market in less time while reducing risks and costs. Partnering with us will help you to get the registration certificate for your product. Our experience includes FDA 510(k) submission and pre-submission, TFDA registration, CE marking preparation, etc.
【Services】
According to specific product and regulatory requirements, we provide systematic and strategic services to lead your product go to market more quickly and successfully, including:
- Consulting of compliance requirements
- Regulatory analysis and marketing strategy
- Training courses in regulatory affairs: Product Registration & Quilaty management system
- Regulatory submission and docissier building, ex: General Safety and Performance Requirements, Risk Management , Clinical Evaluation, Software Aalidation, etc.
- Post-Market Surveillance Planning
【Contact】
Wendy Ho +886-3-591-2520|E-mail: Wendy_Ho@itri.org.tw
Taiwan Integrated Biomedical Industrial Center (TIBIC)
The first experimental field of smart medical cross-domain integration is in Taiwan. With three major verification laboratories and four major simulated clinical fields, combined with innovative technology, we are leading the establishment of an international medical and technology cross-field dialogue platform. With the three characteristics of "verifiable", "intelligent" and "ecosystem", the industry can quickly find product verification opportunities through the field, and adjust and modify products. Doctors can also learn about the latest medical technology and give feedback. Provide counseling usability report for middle and high-end medical devices when applying for marketing permits to shorten the timeline for medical device R&D and marketing.
Establish cross-disciplinary opportunities for practitioners and physicians to make diagnosis and treatment more accurate and efficient. Improve the quality of telemedicine and home care. It can also stimulate technological innovation cooperation and find more new value and market opportunities.
【Contact】
Ada Cheng +886-3-591-2170|E-mail: Adacheng@itri.org.tw
Talent Training
The Leading Learning Service Provider
Developing Innovation Talent for the Next Era
Human capital is a business' most important asset and the force that drives company growth. Learning is the most valuable investment that a business or individual can make. ITRI attaches a great deal of significance to human capital and as such, promotes high quality learning services, enabling both businesses and individuals to unlock their innovation potential through continuous learning. With both market and learning needs in mind, ITRI integrates resources into ITRI's Innovation Competencies and Technology Domains, a program series designed to enhance the innovation capabilities of industrial personnel. ITRI’s learning programs stand out among other education and training institutes because of the features below.
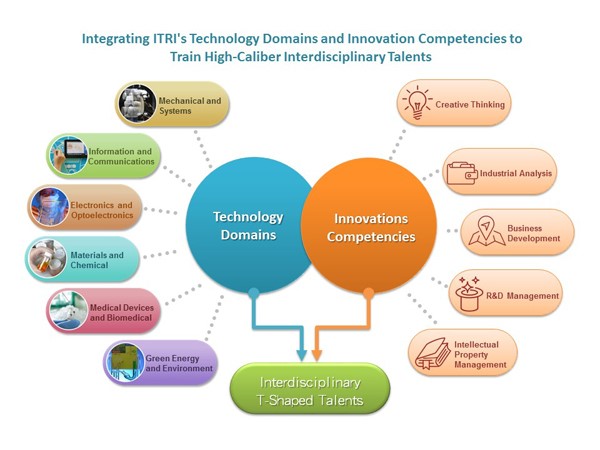
Competency-based Training

Bringing Together Technology Training and Certification
In addition to its in-depth curriculum, built on a foundation of competency requirements for technology professions, ITRI incorporated certifications into its training. Based on the professional competencies of technology specialists, ITRI has developed competency-based certification programs and trained certified seed instructors. These competency models for technology professions also serve as the reference base for corporate training programs.
Innovation Competencies:A Must for Company Managers
The Innovation Competencies program satisfies the different requirements of businesses at various stages of the technology or product development processes. As for the S-curve for business and product development, learning provided by the Innovation Competencies program can inculcate personnel with the skills needed at each and every phase—from idea inception, market and customer analysis, R&D know-how acquisition, application of intellectual property rights to commercialization and the development of new ventures.
Technology Domains

Advancing Interdisciplinary Integration
ITRI’s R&D of technology can be summed up into the following major technology domains—information and communications, electronics and optoelectronics, material, chemical and nanotechnology, green energy and environment, mechanical and systems, as well as medical device and biomedical technologies. ITRI has organized its vast wealth of specialized knowledge in various technology domains into education and training materials. It also offers a number of technology programs each year to provide the human resource training needed by the industry.
Professional Learning Programs
In addition to open enrollment programs, ITRI’s professional team in instructional design is skilled at customizing programs for special learning needs. ITRI services not only domestic and international businesses, but also academia, research organizations, and government organizations, providing learning programs tailored to the special needs of the industry.
For more detail please visit: https://college.itri.org.tw/en/
Open Lab
Industrial Technology Research Institute’s current R&D focuses on 6 key technologies including Information & Communications, Electronic & Optoelectronic System, Material & Chemical, Mechanical & Mechatronics System, Biomedical Technology & Device, and Green Energy & Environment. The goal of the Open Lab is to gather experts in each field both internally from within ITRI and externally to plan long-term technology development strategies as well as promote integration of multidisciplinary technology. Looking forward to the future, ITRI Open Lab aims to expand and transform our operations into a research park to continually elevate ITRI’s superior service to Taiwan industries.
Who can apply?
- Registered company in Taiwan (Foreign Entity Allowed)
- Joint R&D Contract with ITRI Labs/Centers
Incubator
Incubation Center in Hsinchu (Head Office)
ITRI’s Incubation Center in Hsinchu is the first incubator founded in Taiwan. Since its establishment in 1996, the center has taken on the mission of offering assistance to high-tech startups. In August 2013, ITRI's Incubation Center obtained certifications from NBIA softlanding and EBN BIC.
Who can apply?
- Registered company in Taiwan
- Registered company within 5 years and capital under 80 million
Incubation Center in Nankang
Nankang IC Design Incubation Center (NKIC) is a rolling admission acceleration center which focuses on IC design, IoT and any ICT related technology based companies. In addition, NKIC provides highly selected members with value-added services, such as IT, EDA on demand, measurement lab, fundraising support, corporate governance & financial structure planning, IPR strategy & practice, and international marketing channel.
The NKIC has been established for over 13 years and incubated more than 40 companies, over half of which were from abroad, especially Silicon Valley. As a prestigious incubator in Taiwan, NKIC offers complete business assistance in all stages for startups, helping them enhance competitiveness for sustainable growth.
Incubation Center in Tainan
Southern Taiwan Innovation and Research Park (STIR) was established in 2004 and located in Tainan Technology Industrial Park. STIR is a combination of R&D institute (Industrial Technology Research Institute, Institute of Information Industries, and Food Industry Research and Development Institute), open lab, and incubation center. Our mission in STIR is to assist industrial innovation and transformation by providing a new operation model. We are also aiming for balancing regional development in Southern Taiwan, fulfilling local company’s demands and needs, incubating Southern Taiwan’s R&D capability and facilitating innovations.
In STIR, startups could benefit from: the promotion with full functional assistance, cost-savings for new venture, low risk of R&D and enhanced R&D collaboration. Not only can startups gain comprehensive attainment of technological and industrial information but they can also utilize ITRI’s abundant research knowledge as well as international network connections. Also, enterprises are able to improve their value and corporate image.