AERO PRO CO., LTD.
No. 90, Xingong 6th Rd., Tianzhong Township, Changhua County, Taiwan (R.O.C)
About us
ABOUT US

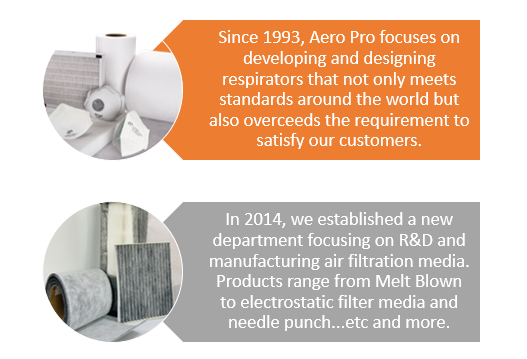
AERO PRO focuses on manufacturing and designing face masks & respirators in Taiwan since 1993. We hope our customers can rely on us full-heartedly no matter what they are facing in their working environment.
We provide not only extra protection for every breath but also each brave and heroic step they take to protect others.
AERO PRO is ISO 9001 & ISO 13485 Certified, also we provide a wide range of products that can meet all different kinds of standards within different countries.
PRODUCT RANGE
1. NIOSH N95 RESPIRATORS
2. NIOSH & FDA CERTIFIED SURGICAL N95 RESPIRATORS
3. EN149 FFP1/ FFP2/ FFP3 RESPIRAOTRS
4. JAPAN JIS DS1/ DS2
5. MEDICAL/SURGICAL FACE MASKS (ASTM F2100/EN14683 STANDARDS)
QUALITY MATTERS
AERO PRO also set up an in-house QA laboratory for our own products to ensure every batch of our products is strictly controlled with superior quality based on ISO 9001 & ISO 13485.

We also manufacture air filtration media that can be used in both medical and industrial settings under our ISO 9001 Quality Control System.
ABOUT US

AERO PRO focuses on manufacturing and designing face masks & respirators in Taiwan since 1993. We hope our customers can rely on us full-heartedly no matter what they are facing in their working environment.
We provide not only extra protection for every breath but also each brave and heroic step they take to protect others.

AERO PRO is ISO 9001 & ISO 13485 Certified, also we provide a wide range of products that can meet all different kinds of standards within different countries.
PRODUCT RANGE
1. NIOSH N95 RESPIRATORS
2. NIOSH & FDA CERTIFIED SURGICAL N95 RESPIRATORS
3. EN149 FFP1/ FFP2/ FFP3 RESPIRAOTRS
4. JAPAN JIS DS1/ DS2
5. SURGICAL FACE MASKS (ASTM F2100/EN14683 STANDARDS)
QUALITY MATTERS
AERO PRO also set up an in-house QA laboratory for our own products to ensure every batch of our products is strictly controlled with superior quality based on ISO 9001 & ISO 13485.

We also manufacture air filtration media that can be used in both medical and industrial settings under our ISO 9001 Quality Control System.

Products
AP0028
● NIOSH N95 approval (Approval number: TC-84A-4175)
● Surgical N95 Respirator approved by FDA 510K
● Low breathing resistance and long lifetime for use
● Two polyurethane headbands secure the respirator to the face to help ensure a tight
facial seal and prevent leakage around the mask edges
● ASTM level of fluid resistance on the market (160mmHg)
● At least 95% filtration efficiency against non-oil based particle
3D Medical Face Mask
● Provide 360 degrees protection
● 3D structure: VF series and TF series
● Meet ASTM F 2100 Level 1
● Adjustable nose wire provide a custom secure seal and soft elastic band provide long
lifetime for use
● Bacterial Filtration Efficiency (BFE) > 99%
● Particle Filtration Efficiency (PFE) > 99%
● Chlid size is avavbile of VF series
Medical / Surgical Face Mask
● USA FDA 510K Approved Surgical Masks
● Meet ASTM F 2100 Level 1 - 3 and EN14683 Type I, Type II, Type IIR
● Fluid-resistant surface to resist blood penetration, soft inner layer that is sensitive skin
friendly
● Bacterial Filtration Efficiency (BFE) > 99%
● Particle Filtration Efficiency (PFE) > 99%
● Pass 160 mmHg Blood Pentration Resistance
Tri-foldable Type Respirator
● Unique design: Tri-foldable Type Respirator
● EN149:2001 CE2797&UKCA0086 approved
● Mark "D": pass dolomite clogging test
● EN149:2001 FFP2 NR D Model lists: AP22102
AP22102V (with valve)
AP22102CV (with valve and activated carbon)
● EN149:2001 FFP3 NR D Model lists: AP22103V (with valve)
AP22103CV (with valve and activated carbon)
AP0708
● NIOSH N95 approval (Approval number: TC-84A-4990)
● At least 95% filtration efficiency against non-oil based particle
● Foldable design provides convenient storage and protability
● The non-latex elastic bands do not irritate or require prestretching
● Low breathing resistance and long lifetime for use (8 hour per shift)
● Adjustable noseclip provide a custom secure seal
AP0018
● NIOSH N95 approval (Approval number: TC-84A-4049)
● Surgical N95 Respirator approved by FDA 510K
● Adjustable noseclip and nose sponge foe fewer pressure points
● Two cloth straps desgin with dual point attachment helps provide a secure seal
● ASTM level of fluid resistance on the market (120mmHg)
● At least 95% filtration efficiency against non-oil based particle
Medical face mask with activated carbon
● Meet ASTM F 2100 Level 2 and EN14683 Type IIR
● Load with high performace actived carbon pre-filter against nuisance organic vapor
● Bacterial Filtration Efficiency (BFE) > 99%
● Particle Filtration Efficiency (PFE) > 99%
● Pass 120 mmHg Blood Pentration Resistance
Activated carbon filter media
● High-quality activated carbon is designed to absorb odors, and VoCs. Can be laminated
to stiff carrier or mesh for pleating purposes or other filtration media to increase
filtration efficiency.
● With high activity coconut carbon for formaldehyde and organic gases remove.
● Can be laminated with other materials, such as melt blown or electrostatic filtration media.
Air filtration media
● Regular melt-blown media that has filter efficiency around 60 - 95%. Used often in
respirators, medical face masks, medium-grade filters...etc.
● For your convenience, it can be laminated to other materials you might need, such as
carbon filter, stiff non-woven media, or electrostatic media... etc.
● Standard product spec sheets are available upon require.